Additional activities
National & international expertise in the viral etiology of sudden cardiac death and myocarditis
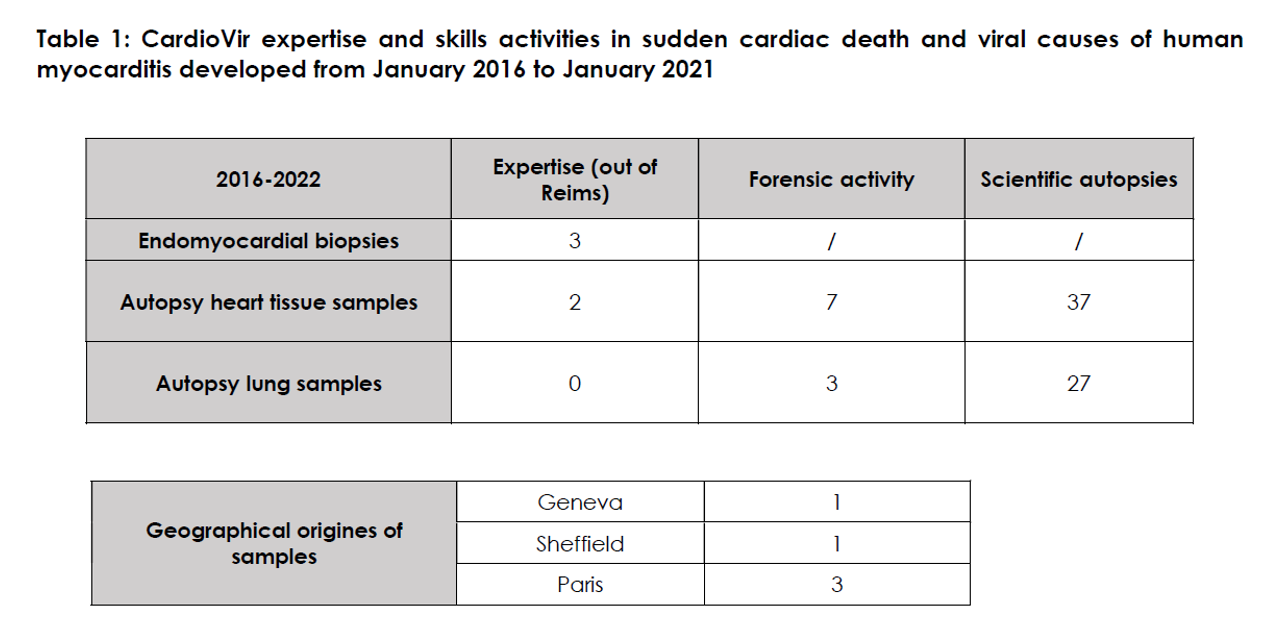
Since 2012, the CardioVir research laboratory has developed and clinically validated quantitative molecular biology tests (taqman-type quantitative RT-qPCR, microarrays...) enabling the detection of common cardiotropic viruses in cardiac tissue derived from ventricular endomyocardial biopsies (EMB) in young living hospitalized subjects, or from post-mortem cardiac samples in subjects who have died of suspected MSC (Ref: https://www.intechopen.com/chapters/21880). These analyses on frozen tissues or formalin-fixed, paraffin-embedded tissue blocks are complemented by histological/immunohistochemical analyses to identify viral protein markers (viral capsid proteins) and inflammatory markers (CD3, CD68, HLA-DR). These samples are autoptic cardiac samples and BEMs (external opinions), in cases of sudden cardiac death (SCD) and fulminant myocarditis, particularly in young subjects and children, in order to clarify the role of viruses in determining SCD and to better define the prevalence of viral myocarditis.
We intervene in complex cases (sudden death sine materia, fulminant myocarditis under immunotherapy...), requiring a precise virological or even genetic diagnosis. We carry out these analyses free of charge, for diagnostic and research purposes. These analyses have led to national (Paris, Lyon...) and international (Sheffield, England; Madrid, Spain...) collaborations, as well as the publication of several articles (clinical cases or cohorts).
Diagnoses are then communicated to the referring clinician and passed on to the patient or family, enabling appropriate screening or prevention to be organized.
Regional expertise in the detection and typing of SARS-CoV-2
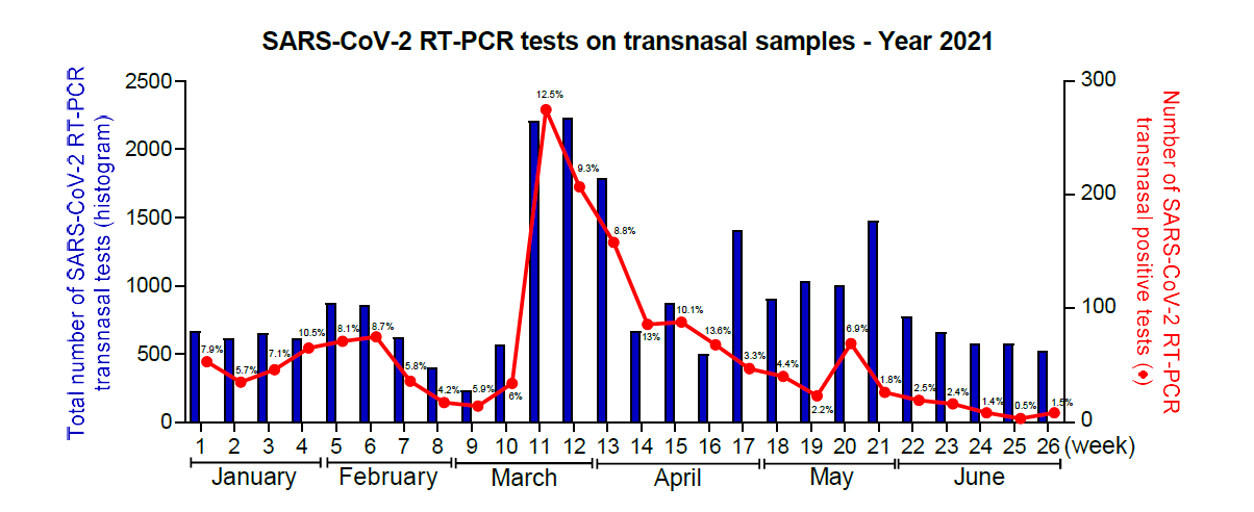
In response to the health crisis caused by SARS-CoV-2 and as part of the 1st decontamination in June 2020, the University of Reims Champagne-Ardenne (URCA) signed agreements with the Reims University Hospital and subsequently with two groups of medical analysis laboratories (Unilabs and Bioxa), under prefectoral dispensation, enabling the setting up of a laboratory approved by the ARS and the Prefect of the Marne headed by Pr. Laurent ANDREOLETTI within EA-4684. The diagnostic laboratory was in operation from June 2020 to July 2021, running so-called high-throughput screening (500 to 2,500 SARS-CoV-2 molecular detection tests) from Monday to Saturday, thanks to the mobilization of CardioVir staff and URCA volunteers (19-25 people), and is capable of performing 1,500 SARS-CoV-2 RT-PCR tests per day.
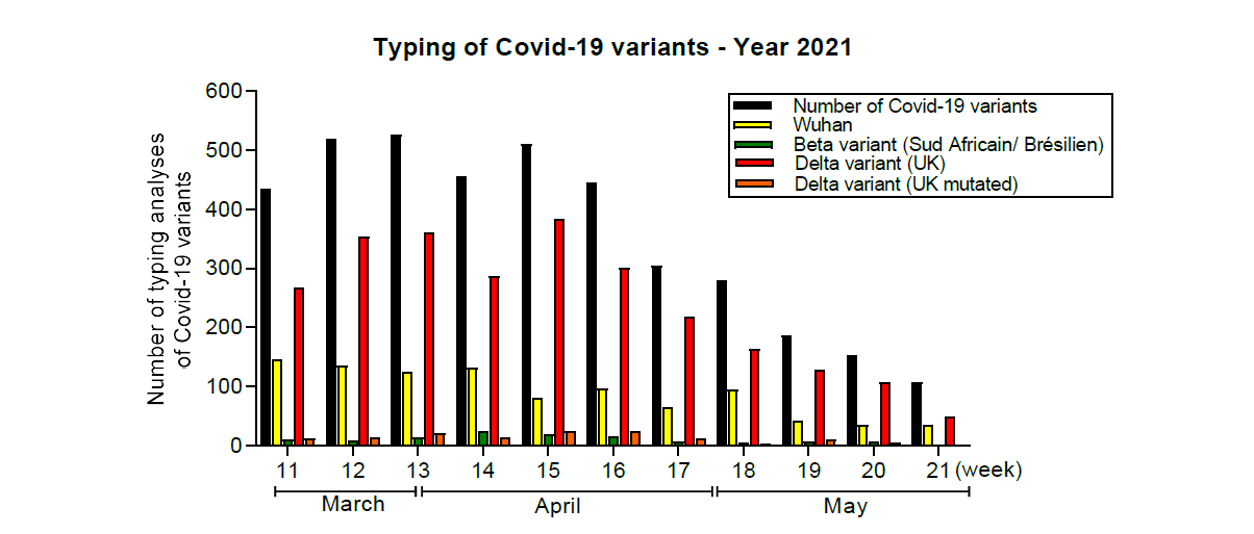
As of March 15, 2021, the laboratory performed COVID-19 variant typing tests on positive specimens, distinguishing Beta (South African/Brazilian) and Delta (UK or UK mutated) variants from the original Wuhan strain. Since June 2021, the laboratory has had a highly performance NGS platform for whole genome or Spike gene sequencing of SARS-CoV-2. Our laboratory has usefully submitted 41 full-length SARS-CoV- 2 genome sequences on EMERGEN-DB platform in order to contribute to the genomic surveillance of SARS-CoV-2 in France through NGS sequencing. Currently, this structural laboratory organization remains on health alert to continue the SARS-CoV-2 screening or genotyping assays.